acetic acid and naoh reaction|is acetic acid a strong acid : iloilo Balanced equation for acetic acid CH 3 COOH with Sodium hydroxide NaOH. When Acetic acid CH 3 COOH reacts with Sodium hydroxide NaOH, the formation of S odium acetate . Sarah Estanislau. contatos, redes sociais e conteúdos 🧜♀️. Onlyfans 🔥. FanFever 🔥. Close Friends (TELEGRAM)
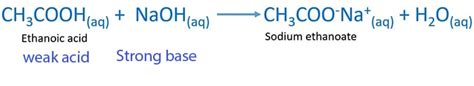
acetic acid and naoh reaction,Explanation: Acetic acid, CH3COOH, will react with sodium hydroxide, NaOH, to produce sodium acetate, CH3COONa, and water. The unbalanced chemical equation that describes this neutralization reaction looks like this. CH3COOH(aq) .Explanation: acetic acid (ethanoic acid): CH 3COOH. CH 3COOH = C2H 4O2. .
Ethanoic (Acetic) acid and NaOH Reaction, pH change, Titration. Aqueous ethanoic (acetic) acid is a carboxylic acid. Rection of ethanoic acid and aqueous NaOH .Balanced equation for acetic acid CH 3 COOH with Sodium hydroxide NaOH. When Acetic acid CH 3 COOH reacts with Sodium hydroxide NaOH, the formation of S odium acetate . In this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. A titration involves performing a controlled reaction between a solution of known .Acetic acid, $$CH_3COOH$$, will react with sodium hydroxide, NaOH, to produce sodium acetate, $$CH_3COONa$$, and water. The unbalanced chemical equation that .The reaction of Sodium hydroxide and Acetic acid (also called Ethanoic acid) represents a net ionic equation involving a strong base and a weak acid. Strong bases are .If you took two ethanoic acid molecules and removed a molecule of water between them you would get the acid anhydride, ethanoic anhydride (old name: acetic anhydride). .Common weak acids include HCN, H 2 S, HF, oxoacids such as HNO 2 and HClO, and carboxylic acids such as acetic acid. The ionization reaction of acetic acid is as follows: \[ CH_3 CO_2 H(l) \overset{H_2 . Acetic acid (CH 3 COOH), the ingredient of vinegar, is a simple example of a carboxylic acid. The K a of acetic acid is 1.8×10-5. Another common organic acid is the organic derivative of sulfuric acid H 2 SO 4. . For the acid-base reaction between C=O group and the proton, the arrow starts from the electron pair on O, .20.18: Reactions of Anhydrides. Page ID. T his page explains what acid anhydrides are and looks at their simple physical properties such as boiling points. It introduces their chemical reactivity in a general way. A carboxylic acid such as ethanoic acid has the structure: If you took two ethanoic acid molecules and removed a molecule of water . H+(aq) + OH−(aq) ⇋ H2O(l) H ( a q) + + O H ( a q) − ⇋ H 2 O ( l) When a strong acid and a strong base fully neutralize, the pH is neutral. Neutral pH means that the pH is equal to 7.00 at 25 ºC. At this .

Acetic acid reacts with aqueous sodium hydroxide and give sodium ethanoate and water as products. Sodium ethanoate is a weak basic salt.ethanoic acid and So.

There are three main steps for writing the net ionic equation for Acetic acid and Sodium hydroxide. First, we balance the molecular equation. Second, we writ.
acetic acid and naoh reaction is acetic acid a strong acid There are three main steps for writing the net ionic equation for Acetic acid and Sodium hydroxide. First, we balance the molecular equation. Second, we writ.
The reaction of aldehydes and ketones with ammonia or 1º-amines forms imine derivatives, also known as Schiff bases (compounds having a C=N function). Water is eliminated in the reaction, which is acid-catalyzed and reversible in the same sense as acetal formation. The pH for reactions which form imine compounds must be carefully controlled.acetic acid and naoh reaction Neutralization Reactions and Net Ionic Equations for Neutralization Reactions. A neutralization reaction is a reaction in which an acid and a base react in an aqueous solution to produce a salt and water. The aqueous sodium chloride that is produced in the reaction is called a salt. The reaction of the weak acid, acetic acid, with a strong base, NaOH, can be seen below. In the reaction the acid and base react in a one to one ratio. C2H4O2 ( aq) + OH − ( aq) → C2H3O − 2 ( aq) + H2O ( l) In this reaction a buret is used to administer one solution to another. The solution administered from the buret is called the . Suppose that we now add 0.20 M NaOH to 50.0 mL of a 0.10 M solution of HCl. Because HCl is a strong acid that is completely ionized in water, the initial [H +] is 0.10 M, and the initial pH is 1.00.Adding NaOH decreases the concentration of H + because of the neutralization reaction: OH − + H + ⇌ H 2 O (in part (a) in Figure 16.5.2).Thus the . The conj. base of acetic acid has a formal charge of -1, so the sodium move closely to it. At the end of the video you show acetone reacting with the hydronium ion. Why does the resultant conjugate acid have a C=OH (double bond between the C and . In this video we'll balance the equation NaOH + HC2H3O2 = NaC2H3O2 + H2O and provide the correct coefficients for each compound. To balance NaOH + HC2H3O2 =.Initially the pH is due to pure acetic acid . As sodium hydroxide is added it reacts with the acetic acid forming its conjugate base, the salt sodium acetate. This is an acetic acid/acetate buffer and the pH is .
Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 14.6.1 14.6. 1 ). A solution of acetic acid ( CH3COOH CH 3 COOH and sodium acetate CH3COONa CH 3 COONa) is an example of a buffer that consists of a weak acid and its salt.
In the titration of acetic acid and NaOH, the use | Chegg.com. 1. In the titration of acetic acid and NaOH, the use of the color indicator mandates that excess base present to detect the reaction endpoint. Will this cause the calculated and reported molarity of acetic acid in vinegar to be slightly high or low?
Benzoic acid and NaOH reaction mechanism. The hydrogen atom in the -OH group of benzoic acid is attacked by the OH - of NaOH to form the H 2 O molecule. Then -OH hroup of benzoic acid is broken and bond electrons are gone to the oxygen atom by producing -O - (benzoate ion). Then Na + cation is combined with benzooate anion to form sodium .Write a balanced chemical equation for the given reaction and also classify them: Lead acetate solution is treated with dilute hydrochloric acid to form lead chloride and acetic acid solution. Sodium hydroxide solution is treated with .
acetic acid and naoh reaction|is acetic acid a strong acid
PH0 · titration of vinegar lab answers
PH1 · net ionic equation acetic acid in water
PH2 · is acetic acid a strong acid
PH3 · hc2h3o2 safety data sheet
PH4 · concentration of acetic acid in vinegar
PH5 · complete ionic equation calculator
PH6 · acetic acid with sodium hydroxide
PH7 · acetic acid vinegar solution formula
PH8 · Iba pa